Diamond
- Diamond is the hardest naturally occurring substance found on Earth and has been the most valuable gem for more than 2,000 years.
- It occurs in two types of deposits: igneous rocks of basic or ultrabasic composition and alluvial deposits derived from the primary sources.
- Diamonds are formed in the mantle and brought to the earth’s crust through volcanism. They are composed of pure carbon and have a cubic crystal system with a common form of octahedron.
- India is known for its diamond cutting and polishing business, especially for small-sized diamonds. The Indian diamond industry handles about 80% of the global polished diamond market, with Surat in Gujarat being a major center for the industry.
- Diamonds are not only used for jewelry but also as an industrial material due to their hardness. They are used for making grinding, drilling, cutting, and polishing tools.
- Diamond exhibits high thermal conductivity and electrical resistivity, making it suitable for applications in semiconductors.
Diamond Distribution in India
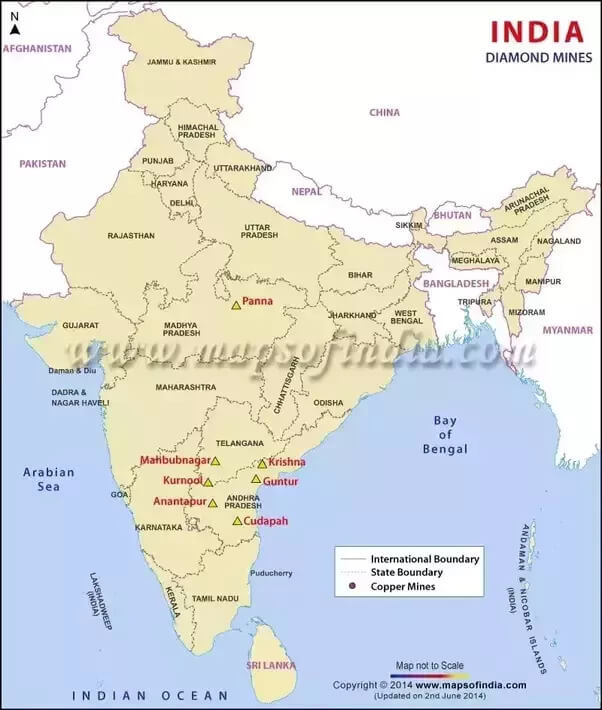
- Diamond occurrences reported in India since prehistoric times
- Diamond fields of India grouped into four regions:
- South Indian tract of Andhra Pradesh
- Comprises parts of Anantapur, Kadapa, Guntur, Krishna, Mahabubnagar, and Kurnool districts
- Central Indian tract of Madhya Pradesh
- Comprising Panna belt
- Behradin-Kodawali area in Raipur district and Tokapal, Dugapal, etc. areas in Bastar district of Chhattisgarh
- Eastern Indian tract mostly of Odisha
- Lying between Mahanadi and Godavari valleys
- South Indian tract of Andhra Pradesh
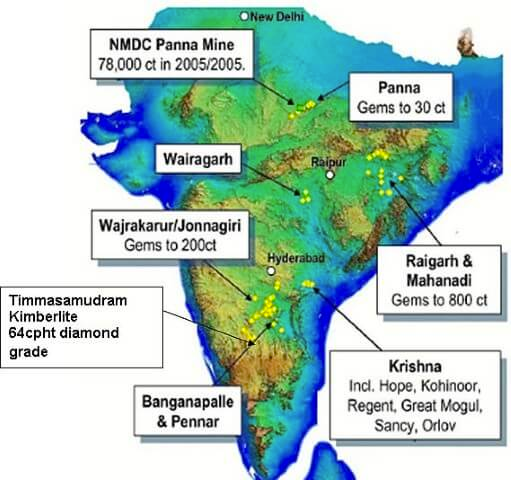
- Reserves estimated only in Panna belt and Krishna Gravels in Andhra Pradesh
- New kimberlite fields discovered in Raichur-Gulbarga districts of Karnataka
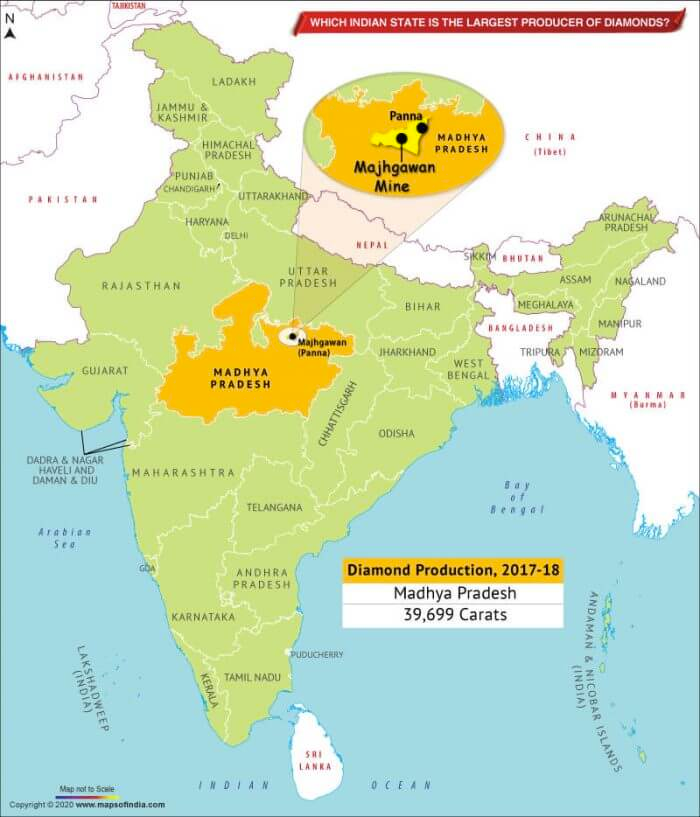
- Madhya Pradesh accounts for about 90.18% of resources
- Andhra Pradesh accounts for 5.72% of resources
- Chhattisgarh accounts for 4.09% of resources
- Only one mine in India located in Majhgaon, Panna (Madhya Pradesh) operated by NMDC
- Mine has a production capacity of 84,000 carats
- Total diamonds recovered from this mine so far are little more than 1 million carats
Graphite
- Graphite is a naturally occurring form of crystalline carbon, also known as plumbago or black lead or mineral carbon.
- It is a stable form of naturally occurring carbon with a carbon content of never less than 95%.
- Graphite is not normally used as fuel because it is difficult to ignite and may be considered the highest grade of coal, just above anthracite.
- It is found in metamorphic and igneous rocks, and most of it is formed at convergent plate boundaries where organic-rich shales and limestones were subjected to metamorphism due to heat and pressure.
- Metamorphism produces marble, schist, and gneiss that contain tiny crystals and flakes of graphite.
- Some graphite forms from the metamorphism of coal seams, known as “amorphous graphite”.
- Graphite is extremely soft, cleaves with very light pressure, and is extremely resistant to heat and highly unreactive.
- It is a non-metal and the only non-metal that can conduct electricity.
Applications of Graphite
- Natural graphite is used for refractories, batteries, steelmaking, expanded graphite, lubricants, etc.
- Refractory materials retain their strength at high temperatures.
- Natural and synthetic graphite are used to make the anode of all major battery technologies.
- Lithium-ion batteries use roughly twice as much graphite as lithium carbonate.
- Natural graphite is used in carbon raising in molten steel to make steel stronger.
- Natural amorphous graphite is used in brake linings for heavier vehicles.
- Graphite lubricants are used at very high or very low temperatures.
- Modern pencil lead is typically made from a mix of powdered graphite and clay.
Indian Graphite Resources
- India’s Graphite occurrences are found in states like Jammu and Kashmir, Gujarat, Jharkhand, Arunachal Pradesh, Karnataka, Kerala, Maharashtra, Tamil Nadu, Odisha, Chattisgarh, and Rajasthan.
- As per GSI’s 2013 report,
- Arunachal Pradesh (43%),
- Jammu & Kashmir (37%),
- Jharkhand (6%),
- Tamil Nadu (5%) and
- Odisha (3%)
- Operational Indian Graphite Resources
- Most of the Graphite Production is concentrated in these states
- Tamil Nadu (37%),
- Jharkhand (30%), [Palamu district in Jharkhand is the most important]
- Odisha (29%).
- Active mining centres of graphite are in
- Jharkhand – Latehar & Palamu districts
- Odisha – Bargarh, Nuapada, Rayagada & Balangir districts
- Tamil Nadu – Madurai & Sivagangai districts
Differences Between Graphite and Diamond
| Diamond | Graphite |
| In diamonds, strong three-dimensional networks are formed due to the presence of covalent bonds. | Graphites are formed due to the weak van der Waals force of attraction. |
| Hard in nature | Soft in nature. |
| Since molecules are closely packed they have high density. | Because of the large gap between the molecules, they have low density. |
| Since there is no free carbon atom, the diamond does not conduct electricity. | Because of the presence of free carbon atoms in graphite, they can conduct electricity. |
| Diamond is 100% carbon. | Graphite contains 95% or more carbon. |
| Diamond (one of the most stable) is less stable than graphite. | Graphite is one of the most stable substances on earth. |

0 Comments